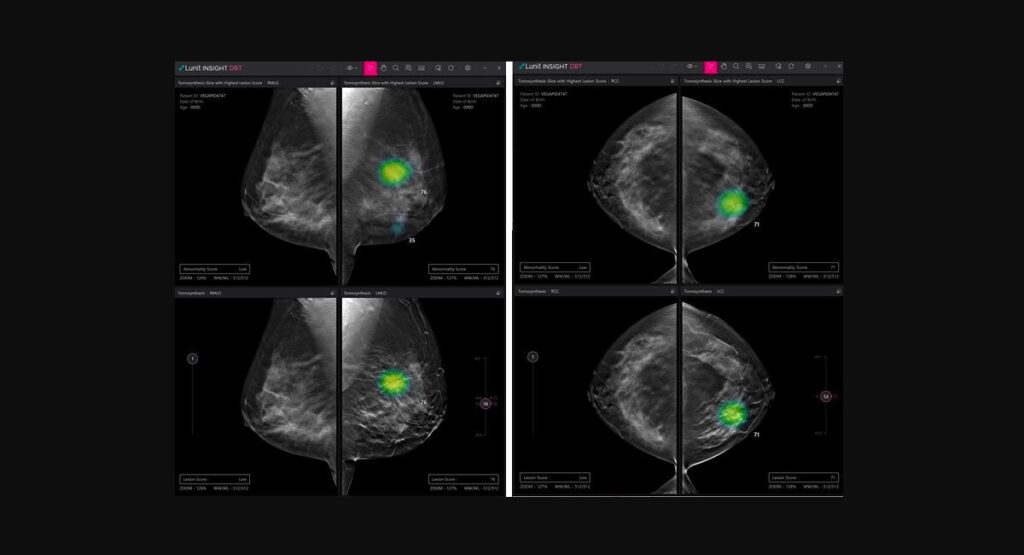
Lunit obtains new CE mark for AI DBT solution
South Korean medical AI company Lunit has received a CE mark under Europe’s latest Medical Device Regulation for its AI software for digital breast tomosynthesis (DBT) analysis.
Called Lunit INSIGHT DBT, the software solution analyses 3D images from DBT, enabling fast and accurate diagnosis of breast cancer.
In a press statement, Lunit disclosed its plan to start rolling out the software product in Europe by the end of March, noting an uptick in interest. It also announced its plan to begin the process of acquiring the US FDA’s approval for Lunit INSIGHT DBT in the third quarter. The technology has already been approved for commercialisation in South Korea early this year.
Jolly Good creates emergency care VR content with Brigham and Women’s Hospital
Jolly Good has revealed its latest collaboration with Harvard Medical School-affiliated Brigham and Women’s Hospital (BWH).
According to a press statement, their partnership seeks to verify the educational benefits of medical VR and its implementation in North America.
As part of their collaboration, the organisations have developed VR content on emergency care.
Meanwhile, the Japanese VR company announced that Dr Kei Ouchi, associate professor at Harvard Medical School, has become its medical advisor who will guide its full entry into the US medical market.
IIT Madras researchers develop coronavirus antibodies database
Researchers from the Indian Institute of Technology Madras (IIT Madras) have built an open-source database of coronaviruses’ antibodies.
Called Ab-CoV, the online database includes antibody features such as binding affinity and neutralisation profiles, source, and identifying viral proteins and strains. Currently, Ab-CoV has data on 1,780 coronavirus-related antibodies and contains more than 3,200 data points on their features.
“Ab-CoV is an exhaustive repository of antibodies, not just specific to SARS-CoV-2, but also to other members of the coronavirus family, such as SARS and MERS viruses,” Dr Vani Janakirama of the Department of Biotechnology explained.
Based on a press statement, the Ab-CoV database can be used to aid the development of new drugs against emerging variants of SARS-CoV-2, the virus which causes COVID-19.
“This repository would aid in comparative studies among different neutralising antibodies across coronaviruses and assess their properties and interaction patterns with epitopes on the native and mutant viral proteins. Such an effort eventually would help to gauge the efficacy of these antibodies towards existing and emerging viral variants,” Dr Janakirama added.