May 23, 2023 – Imagine a day when a simple injection prompts a broken bone to heal. When tiny, ingestible devices linger in the body, unnoticed, tracking our health or delivering life-saving medications. When brain and heart implants mesh with flesh so seamlessly that the body thinks they’ve been there all along.
These are the dreams of materials scientists who have toiled for decades to mimic the complex architecture of the human body in hopes of replacing broken parts or treating disease.
The problem, say bioengineers, is that most replacement and corrective parts – from prosthetics to pacemakers – are made of hard, dry, lifeless materials, like metal or plastic, while biological tissue is soft, wet, and living.
The body knows the difference and tends to reject imitations.
Enter hydrogels, three-dimensional networks of molecules swollen with – by definition – water.
First described in 1960 by creators of soft contact lenses, these weird, shape-shifting substances are able to morph from liquid to solid to a squishy in-between. (Early, simple uses include hair gel or Jell-O.). Slow to gain attention, growing to just 1,000 studies published by 1982, they’ve become the subject of intense study recently, with 100,000 papers total published by 2020, and 3,800 already this year alone.
As chemists, biologists, and engineers begin to work more with one another and with medical doctors, the burgeoning hydrogel field is poised to transform the way we take medication and treat worn-out joints and pave the way for a seemingly sci-fi future in which organs, including brains, can interact directly with machines.
“We are, essentially, hydrogels,” said Benjamin Wiley, PhD, a chemistry professor at Duke University in Durham, NC. “As people develop new hydrogels that more closely match the tissues in our body, we’ll be able to treat a whole host of ailments we couldn’t treat before.”
From Contact Lenses to Brain Implants
Put simply, a hydrogel is like a mesh bag of water.
The mesh is made of polymers, or spaghetti-like strands of molecules, stitched together in a repeating pattern and swollen with H2O, much like the way 3D matrixes in our body surround, support, and give structure to our cells and tissues.
“Imagine a soccer net, with all of these long fibers woven together to create the net,” said Eric Appel, PhD, an associate professor of materials science and engineering at Stanford University.
While the broader category of “gels” could be filled with anything, including chemical solvents, water is the key ingredient that sets hydrogels apart, making them ideal for, as some scientists put it, “merging humans and machines.”
Human bones are about 25% water, while muscles hover around 70% and the brain is 85%. The precious liquid plays a host of critical roles, from shuttling nutrients in and waste out to helping cells talk to each other.
Lab-made hydrogels can be loaded with cargo (like a ball in the net), including cells or drugs that help mimic some of those functions.
Hydrogels are also soft and pliable like flesh. So, if used in implants, they may be less likely to damage surrounding tissue.
“Think about a metal spoon in your bowl of pudding. As you’re shaking the bowl, the spoon doesn’t stay in place, and you get scarring around the spoon,” said Christina Tringides, PhD, a materials scientist who studies neural engineering. That, she says, is exactly what happens to brain implants when patients breathe or move. “It’s a mechanical mismatch. But with hydrogels, you could get perfect mechanical matching.”
Hydrogels also tend to be nontoxic, so the immune system may be less likely to attack them as foreign bodies.
All this has made hydrogels the new darling of the bioengineering world.
“There has been an absolute explosion of interest in these materials,” Appel said.
Smarter Drug Delivery and Ingestible Electronics
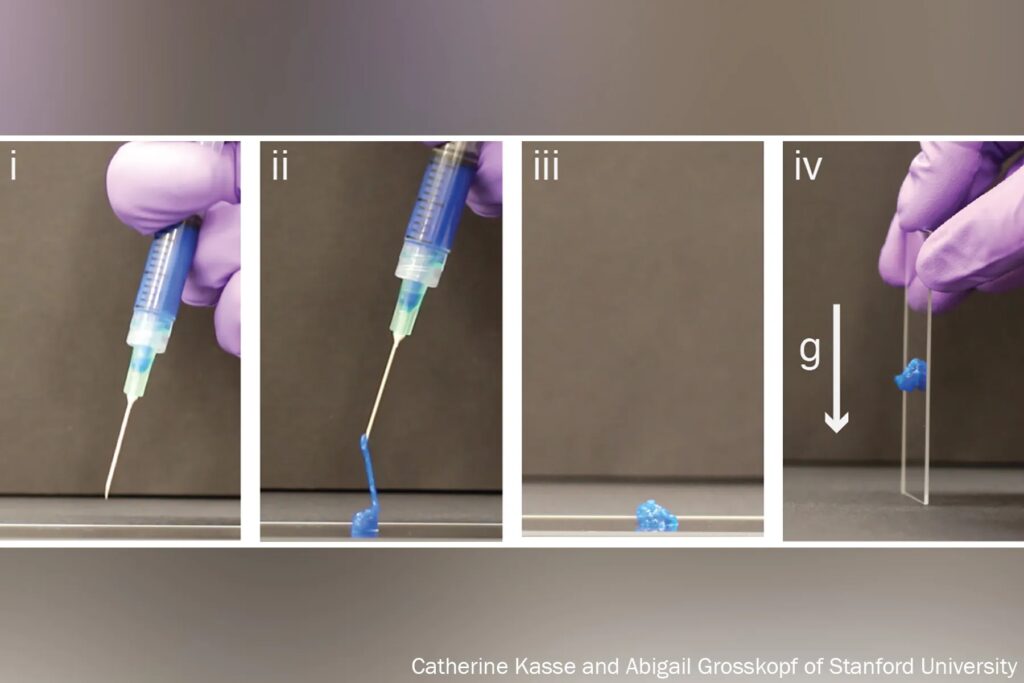
Early versions of hydrogels were thick and gooey, making it hard to get them inside the body.
“Think of a block of Jell-O. You couldn’t inject something like that,” Appel said.
But Appel, whose lab develops new drug delivery systems, has been tinkering with gel formulas for years in hopes that these high-tech globs could someday ferry timed-release drugs to just the right spot in the body.
His new hydrogels start as fully formed gels (which help preserve the drug contents) inside a syringe. But once the plunger is pushed, they magically shape-shift to a liquid thin enough to flow easily through a standard needle. Upon exit, they immediately reform into gels, protecting the inherent cargo from degrading.
This could be a game changer at a time when many cutting-edge drugs – think Humira for arthritis or Ozempic for type 2 diabetes – are made of quickly degrading proteins too large and complex to simply jam into a pill. Instead, they must be injected, often frequently.
“Because the gel takes months to dissolve, it slowly delivers the drug over time,” Appel said. “You could conceivably go from a shot once a week to once every 4 months.”
Such slow-release hydrogels could make vaccines last longer, in turn teaching the body to better resist emerging virus variants, and deliver tumor-busting therapies more precisely, said Appel, who has formed a startup and hopes to fast-track the first hydrogel drug delivery system to clinical trials within a few years.
Meanwhile, another team at the Massachusetts Institute of Technology has taken a different approach, developing a standard-sized ingestible hydrogel pill that swells up like a puffer fish in the stomach, lasting a month and slowly releasing drugs all the while. To remove the pill, a patient simply drinks a salt-based solution that shrivels the ping-pong ball-sized device so it can be passed out of the body.
In a paper in Nature Communications, the scientists showed the puffer fish pill could also be loaded with tiny cameras or monitors to track conditions like ulcers or cancer.
“The dream is to have a Jell-O-like smart pill that, once swallowed, stays in the stomach and monitors the patient’s health,” said Xuanhe Zhao, PhD, a researcher on the project and an associate professor of mechanical engineering at MIT.
Building Joints and Regrowing Bones
Since the 1970s, researchers have mulled using hydrogels to replace human cartilage, a remarkably strong and flexible tissue made of about 90% water but able to withstand the weight of a car on an area about the size of a coin.
Until recently, those efforts have largely failed. Meaning when knee cartilage wears down, things like cartilage transplants, drilling holes to stimulate new growth, or total joint replacements – all of which require lengthy rehab – are the only options.
But that may be about to change.
Wiley and his colleagues at Duke recently reported that they’d developed the first gel-based cartilage substitute even stronger and more durable than the real thing.
By attaching their hydrogel to a titanium backing to help stick it in place, they hope to repair damaged cartilage “much like a dentist fills a cavity” long before surgery is necessary.
They too have partnered with industry to bring their hydrogel to market – starting with knees.
“Ultimately, the goal is to do any joint – hips, ankles, fingers, and toes,” Wiley said.
At the University of Toronto, chemist Karina Carneiro, PhD, and dentist Christopher McCulloch, DDS, are also thinking big.
In a recent paper in Proceedings of the National Academy of Sciences, they describe a hydrogel, designed by Carneiro and made of DNA, that can be injected, migrate to a defect in bone — an irreparable break, hole from surgery, or jawbone withered by age — and fill in the gap like putty. But not only does it patch the hole, it prompts the bone to regenerate.
In rats with holes in their skull due to surgery, they found that the treatment did not work as well as the existing gold standard for repairing holes in bone – grafting bone from elsewhere in the body. But it did work.
“These are very early days for DNA hydrogels,” cautioned McCulloch, a study co-author and professor in the Faculty of Dentistry, noting that it will likely be a decade or more before such technology could be available to patients. “But there is the potential that DNA hydrogel could someday grow bone without having to have highly invasive surgical procedures. That’s a significant advancement.”
A Sci-Fi Future
Perhaps the wildest, and weirdest, potential applications of hydrogels come in the realm of human-machine interaction.
Numerous companies are already dabbling in neural prosthetic or brain computer interfaces that might someday, for instance, let someone who is paralyzed and can’t speak write on a laptop using their thoughts.
The spoon-in-the-Jell-O problem has been a major stumbling block.
But Tringides, who recently earned her PhD in biophysics from Harvard, is working on it.
She and her team have developed a seaweed-based hydrogel loaded with tiny flecks of nanomaterials that can not only meld nicely into squishy brain tissue but also conduct electricity.
Within a decade, she says, this could replace the clunky platinum metal discs used for electrocorticography — recording electrical activity in the brain to identify where seizures start or doing precise brain surgery.
In 30 to 50 years? Let your imagination run wild.
“I’m a skeptic. I like to take research step by step,” she said. “But things are definitely progressing in an interesting direction.”