Medtech company Masimo announced it had received FDA De Novo clearance for Opioid Halo, the company’s opioid overdose alert system.
The monitoring system aims to prevent overdose by alerting family members or caregivers when a patient is experiencing opioid-induced respiratory depression, or slowed or stopped breathing that can be fatal.
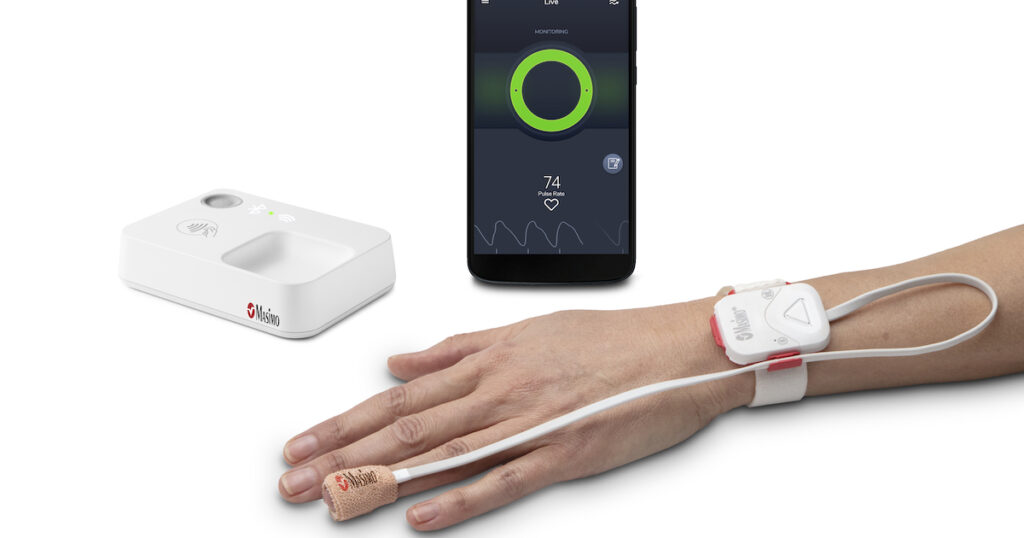
Opioid Halo includes four components: a fingertip sensor, a reusable pulse oximeter, a home medical hub and a smartphone app. The system provides escalating alerts if it detects signs of respiratory depression, including auditory and visual alarms and automated texts to friends and family.
Users can also add an optional setting that allows a service center to make automatic wellness checks if the system detects potential overdose, which could include dispatching emergency services.
Masimo said Opioid Halo is approved for over-the-counter use for patients ages 15 and older. It’s also offering a prescription version of the product.
“We are very excited to be able to offer this solution to our fellow Americans and the community heroes who are helping to battle the opioid crisis – a crisis so devastating in its impact on the young that it has lowered overall life expectancy in the U.S.,” Joe Kiani, founder and CEO of Masimo, said in a statement.
“Now, with Opioid Halo, we hope to help make a big difference by providing a much needed tool that can help millions, whether they are taking prescribed opioids or struggling with illicit opioid use.”
THE LARGER TREND
According to provisional data, overdose deaths dropped modestly in the first nine months of 2022 after spiking during the COVID-19 pandemic. Still, nearly 80,000 died during that time period, and synthetic opioids like fentanyl are driving overdose deaths across the country.
Masimo had been working on the Opioid Halo for several years. The company was included in the FDA Opioid Innovation Challenge, launched in 2018, that aimed to create medical devices, digital health products and diagnostics to address opioid abuse and misuse.
Just last week, the FDA also approved Narcan, a naloxone nasal spray that reverses an opioid overdose, for over-the-counter use.
Howard Rubin will offer more detail during the HIMSS23 session “Increasing Access to Care for Rural and Underserved Communities.” It is scheduled for Tuesday, April 18 at 3 p.m. – 4 p.m. CT at the South Building, Level 1, room S105A.